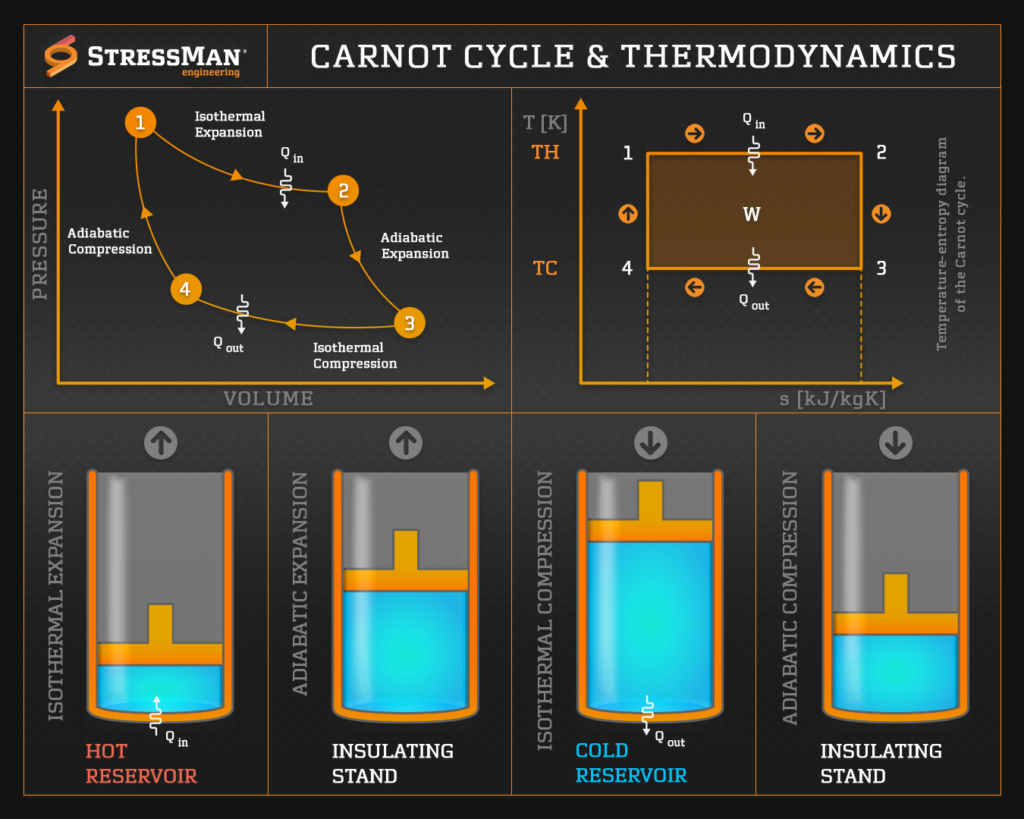
The Carnot cycle is a theoretical thermodynamic cycle that represents the most efficient heat engine cycle possible. It serves as an idealized benchmark for the maximum efficiency that any heat engine operating between two temperature reservoirs can achieve.
The cycle was developed by the French engineer Sadi Carnot in 1824 and is an essential concept in thermodynamics. The Carnot cycle consists of four successive reversible processes:
- Isothermal expansion (process 1-2): the working fluid undergoes expansion while in contact with a high-temperature reservoir (source) at a constant temperature (TH). During this process, heat energy (Qin) is absorbed, and the fluid expands.
- Adiabatic expansion (process 2-3): The fluid continues to expand but now without any heat exchange with its surroundings. This adiabatic expansion results in a decrease in temperature (fluid cools from TH to TC) and pressure.
- Isothermal compression (process 3-4): The fluid is brought into contact with a low-temperature reservoir (sink) at a constant temperature (TC). Heat energy is rejected from the fluid (Qout), causing it to contract.
- Adiabatic compression (process 4-1): The fluid is further compressed, but now without any heat exchange. This adiabatic compression raises the temperature (fluid heats from TC to TH) and pressure of the fluid, bringing it back to its initial state.
Net work (Wnet) done by the process equals to the difference between heat input (Qin) and heat output (Qout).
Wnet = Qin – Qout
By combining the first and second laws of thermodynamics, the efficiency of the Carnot cycle (denoted as ηCarnot) which is defined as the ratio of the net work (Wnet) to the heat input (Qin), can be shown to be:
ηCarnot = 1 – TC / TH
in which TC is the absolute temperature of the cold heat reservoir, and TH is the absolute temperature of the hot temperature reservoir.
Notably, the efficiency of the Carnot cycle depends only on the temperatures of the reservoirs and not on the specific working substance or details of the engine design. It represents an upper limit to the efficiency of any heat engine operating between the same two temperatures.
The Carnot cycle is a fundamental concept in thermodynamics and provides insight into the limitations of heat engines. It helps in understanding concepts like entropy, heat transfer, and reversibility. While real-world engines cannot achieve the ideal efficiency of the Carnot cycle, it serves as a valuable benchmark for engineers when designing and optimizing practical heat engines.